Accolades
2018 CAS Research Instrumentation Program
The College of Arts & Sciences launched a new Research Instrumentation program in 2016. The program required combined CAS department contributions to purchase the following research instrumentation:
The Department of Chemistry
The Lab Strong FI-STREEM System:
Instrumentation for Preparation of Ultra-High Purity Reagent Water for Analytical,
Biochemical, Environmental and Materials Chemistry Research
 High purity reagent water is often taken for granted but its importance cannot be
underestimated in the research efforts of the experimentalists in the Department of
Chemistry as well as our colleagues in the molecular cell sciences. Currently, the
Department of Chemistry provides reagent water largely at our own expense for many
researchers in these previously mentioned disciplines. While the quality of the reagent
water we prepare has been acceptable, it could certainly be improved. The Lab Strong
FI-STREEM system is set up and operating in Rm 27 of Smith Chemistry Building. It
greatly improves of reagent water quality from ~18.2 MΩ water to at least 18.6 MΩ.
This may not seem like much of an increase, but it important to explore what these
numbers actually mean.
High purity reagent water is often taken for granted but its importance cannot be
underestimated in the research efforts of the experimentalists in the Department of
Chemistry as well as our colleagues in the molecular cell sciences. Currently, the
Department of Chemistry provides reagent water largely at our own expense for many
researchers in these previously mentioned disciplines. While the quality of the reagent
water we prepare has been acceptable, it could certainly be improved. The Lab Strong
FI-STREEM system is set up and operating in Rm 27 of Smith Chemistry Building. It
greatly improves of reagent water quality from ~18.2 MΩ water to at least 18.6 MΩ.
This may not seem like much of an increase, but it important to explore what these
numbers actually mean.
Resistivity (the value expressed in units of MΩ) is the inverse of the conductance – the ability of a water to conduct current. Water that is 18.2 MΩ is typically considered "pure water", but what this means is that this water is relatively pure of ions and thus does not efficiently conduct current. However, this measure of 18.2 MΩ does not take into account the "total organic content" of the water in terms of "purity". The fact is that that there are many DNAse and RNase compounds left over from bacteria can potentially complicate bioanalysis. Additionally, in studies of disinfection chemistry, the same compounds can contribute to oxidant demand and skew results. The Lab Strong FI-STREEM system takes "pure" 18.2 MΩ reagent water that we produce already and subjects it to a double distillation process that essentially make our reagent water free from such complicating chemical compounds.
The LabStrong Corp FI-STREEM Bi Distillation system is an extremely high quality automated distillation system capable of distilling 8 liters per hour. This particular system provides for 24-hour automatic control meaning that the still is activated only as needed to maintain a full water storage reservoir. This saves wear and tear on components as well as energy of operation. The system is easily shut down for extended time periods without having to replace costly consumables. Finally, the fully automated controls in FI-STREEM III stills simplify operation while assuring high reliability and the highest quality water. In our case, the "house" water we produce in the penthouse of Smith Chemistry Building will still be used in connection with our current RO systems, but then the water we currently use as reagent water will then be subjected to "double distillation" in the Lab Strong FI-STREEM system.
The LabStrong 40 Liter Storage Tank is not just a water storage tank, it is a key component for fully automatic operation when interfaced to the above FI-STREEM double distiller unit. The tank is formed from high density polyethylene in a space saving, wall mounted design. The tank is constructed with window for observation of the water levels and also with a vent filter that prevents airborne contamination. A spigot is attached for easy dispensing of water. A distribution accessory kit included in the estimate is used to connect the 40 liter tank to a secondary output other than from the spigot at the front of the tank. The distribution accessory kit allows for distribution of distilled product water to point of use deionization (DI) system or any system requiring distilled water as a feed source, while leaving the spigot at the front of the tank free to manually draw water from at any time.
Usage Policy
The LabStrong 50 Liter Carboy allows for fully automatic operation when connected to FI-STREEM double distillation systems. The carboy is formed from low density polyethylene. The portable design is ideal for transporting distillated water to different lab sites. In our case, we are making ultra-high purity reagent water easily accessible to all departmental researchers in Chemistry meaning that at least one carboy will be continuously available on three different floors, with one in reserve near the main unit so that one full carboy is always available for quick changeouts. Other departments will be asked to purchase their own carboys to participate in the program. Included with the reservoir is a spigot for easy dispensing of water. The specialized cap is modified to accept the FI-STREEM Float Switch to prevent overflows. Researchers from other departments simply need to bring an appropriate container (carboy) to Smith Chemistry Building RM 27 during normal office hours or by appointment to collect appropriate volumes of the high purity reagent water. The contact person is Joseph Burns (jburns7@memphis.edu ). Please contact him by email to arrange a fill time.
Department of Physics
Bright/Dark field Nikon microscope system for optical measurements of single nanostructures
Instrument Capabilities and Usage Policy
Location: Room MN 107, Department of Physics and Materials Science
Bright/Dark field microscope system (LV 150 N) was acquired from Nikon Inc. this past Spring semester, 2018. The microscope came with a set of Bright/Dark field objective lenses, including 5X, 10X, 20X, 50X (extra-long working distance), 100X magnifications and a sensitive Photometrics Dyno CCD camera. These objective lenses, when integrated with other optics components in the Nanophotonics Lab, allow one to look at single nanostructures such as single molecules, quantum dots, nanowires or plasmonic nanoparticles from visible to near IR frequency. Besides the white light illumination, the microscope system is also customized with an optical path which enable a tunable laser to optically excite the fluorescence of nanomaterials. Optical signal from the microscope is guided and collected by the camera or by a spectrometer for frequency analysis and time-resolved measurements.
 Figure 1 shows a picture of the microscope installed in the lab along with other optics
which allows external optical excitation and detection. In short, besides having most
functions of a regular microscope (viewing and imaging structures at small scales),
this Bright/Dark field microscope system when integrated with other spectroscopic components
in the Nanophotonics Lab can provide:
Figure 1 shows a picture of the microscope installed in the lab along with other optics
which allows external optical excitation and detection. In short, besides having most
functions of a regular microscope (viewing and imaging structures at small scales),
this Bright/Dark field microscope system when integrated with other spectroscopic components
in the Nanophotonics Lab can provide:
- Single nanostructure imaging
- Single nanostructure fluorescence imaging
- Single nanostructure spectroscopy
- Raman spectroscopy of nanostructures
- Time-resolved fluorescence
- Fluorescence of upconversion nanocrystals
- Antibunching measurements
- Coincidence correlation measurements
- Single molecule spectroscopy
(Figure 1 to the right: Picture of the newly installed microscope, which has been integrated with other optical components in the Nanophotonics Lab (MN 107).)
 This microscope system offers a powerful capability to the optical study of the broad
areas of physics, materials science, chemistry, and biology. Specifically, this system
offers possibilities to study the spectral, spatial and temporal dynamics, photon
statistic and ultrafast optical, electronic processes that occur in novel nanomaterials (including
nanocrystals, semiconductor nanowires and rods, 2-dimensional dichalcogenides), organic materials
(such as organic dyes or photochromic molecules) and ultrafast optical processes in nanophotonic
and plasmonic nanostructures.
This microscope system offers a powerful capability to the optical study of the broad
areas of physics, materials science, chemistry, and biology. Specifically, this system
offers possibilities to study the spectral, spatial and temporal dynamics, photon
statistic and ultrafast optical, electronic processes that occur in novel nanomaterials (including
nanocrystals, semiconductor nanowires and rods, 2-dimensional dichalcogenides), organic materials
(such as organic dyes or photochromic molecules) and ultrafast optical processes in nanophotonic
and plasmonic nanostructures.
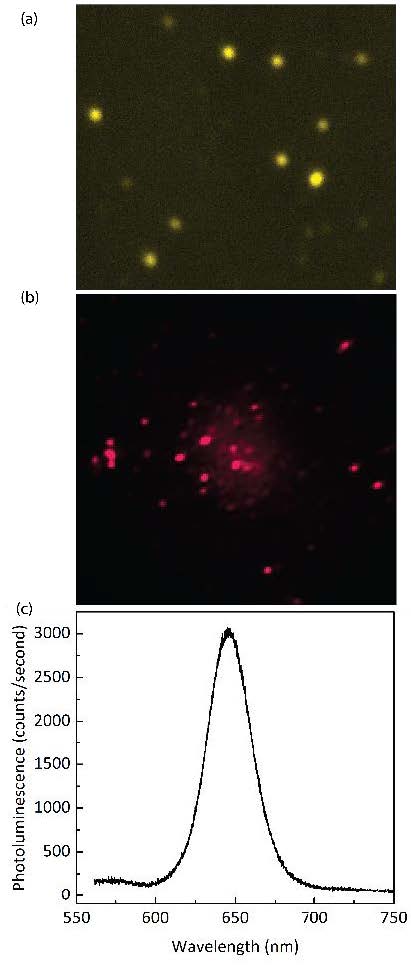
An example of preliminary data taken with this newly installed microscope setup is shown in Figure 2. Figure 2a shows a dark field scattering image of gold nanoparticles. These individual nanoparticles have sizes around 50-70 nm and they become very bright under white light illumination as a result of localized surface plasmon resonances. Figure 2b shows a fluorescence image of CdSe quantum dots when excite with a laser, each individual quantum dot shows up as a bright spot. Figure 2c shows corresponding emission spectrum of the quantum dots displayed in Figure 2b. By using a spatial filter (not be shown here), the microscope system can also measure the spectrum of individual quantum dots.
(Figure 2 to the right. Example of preliminary data taken with the new microscope setup. (a) Image of individual gold nanoparticles under white light illumination. The nanoparticles are approximately ~ 50 nm. (b) Fluorescence image CdSe quantum dots, under 400 nm laser excitation and (c) Emission spectrum of the quantum dots shown in (b).)
Usage Policy
For user access from outside of the Department Physics and Materials Science, no special arrangement is needed. Users will first need to contact appropriate personnel (Presently, Dr. Hoang, email tbhoang@memphis.edu responsible for maintaining of the system) for available time. The system can be used together with other spectroscopic equipment in the Nanophotonics Laboratory, including an ultrafast laser, high spectral, temporal and spatial resolution spectroscopic setups and other polarization optics. Tothis end, the users are required to have some basic training regarding the laser safety and operations, and uses of spectroscopic equipment.
A fee structure is established for use of above facilities for both internal and external users. The fee structure is determined with reference to Integrated Microscopic Center (IMC) on campus as shown below. For those faculty who contributed their own research funds to the purchase of facilities in the Nanophotonic Lab, their contributed funds will be considered as prepaid user fees for future service. The recovered cost will go towards regular maintenance, contract service, and consumable items.
All rates hourly. Fees are charged in whole hour increments with the minimum charge of 1 hour.
| Instrument | Internal User Rates | External User, Non-Profit Organization Rates | External User, For Profit Organization Rates |
| Nanophotonics Lab | $50 | $80 | $100 |
| Technical Assistance | $50 | $80 | $120 |
Surface Area Analyzer
Instrument Capabilities and Usage Policy
Person-in-charge: Prof. Sanjay Mishra, srmishra@memphis.edu
Location: Room MN 117B, Department of Physics and Materials Science
A state-of-the-art surface area analyzer, NOVAtouch LX-2 for Quantachrome Instruments (F L, USA), has been acquired through CAS Research Instrumentation Initiative in March 2018. The system has been successfully installed and tested for its capabilities for measuring surface area, pore size, and pore size distribution of nanopowders. The system has four controlled baking station for removing moisture from samples and two measuring stations for performing controlled adsorption-desorption experiments simultaneously. The system is fully automated and requires minimum user input. All measurements are performed at liquid nitrogen temperature at 77K.
Surface area and porosity are physical properties that impact the quality and character of solid phase materials. Materials with identical physical dimensions may exhibit entirely different performance profiles based on variations in the physical surface area of the two materials.
Surface area measurement if an important analysis used in many research laboratories and industries, including catalysts, zeolites, batteries, absorbents, artificial bone, pharmaceuticals, metal powders for additive manufacturing along with a wide variety of other applications and industries.
The surface area is measured using BET analysis (Brunauer–Emmett–Teller (BET) theory), which provides precise specific surface area evaluation of materials by nitrogen adsorption measured as a function of relative pressure. The surface area is determined by calculating the amount of adsorbate gas corresponding to a monomolecular layer on the surface of the material.
The technique encompasses external area and pore area evaluations to determine the total specific surface area. BET is used to determine a range of disperse, solid microporous to mesoporous materials. In addition, BJH analysis can also be utilized to define pore area and specific pore volumes through adsorption and desorption techniques. Using BJH analysis one can conclude pore size distribution independent of the external area due to a particle size of the sample. Both information, surface area, and pore size analysis of the sample is highly sought information in the field of nanomaterials having varied nanoarchitecture, pores, and surfaces.
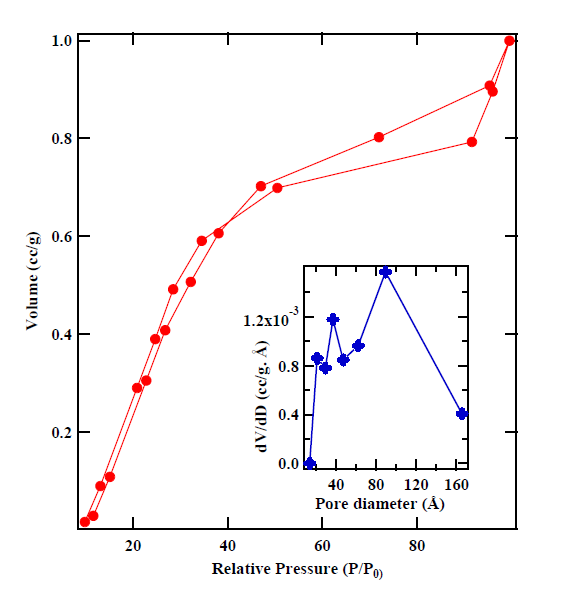
In addition, several other methods are available to measure surface area and to perform pore size analysis in the software. Figure 1 shows absorption-desorption isotherms of cobaltites measured using surface area analyzer. The inset shows pore size distribution as calculated using BJH analysis.
Figure 1a: NOVAtouch LX-2 set-up in MN 117B. Figure 1(b): Absorption-desorption curves of cobaltite. The inset shows pore size distribution as measured using BJH analysis.
|
Figure 1a: NOVAtouch LX-2 set-up in MN 117B. |
Figure 1(b): Absorption-desorption |
User Policy
The surface area analyzer is available for campus and public use. In order to schedule use of the instrument, the user needs to directly contact Dr. Mishra at srmishra@memphis.edu. The system will be available on the first come first serve basis. Prior to using instrument Dr. Mishra will provide initial training to the user. Due to the turn-key nature of the instrument, minimum training for the user is required. A proper user logbook will be maintained for the instrument usage. Data will be secured and will be transferred out of the computer on a secured disk as a part of the yearly maintenance of the equipment.
A fee structure is established for use of above facilities for both internal and external
users. The fee structure is determined in tandem with the Integrated Microscopic Center
(IMC) on campus as shown below. For those faculties who contribute their own research
funds using Dr. Mishra's Nanomaterials Research Facility, will be considered as a
prepaid user for the future service. The
recovered cost will go towards regular maintenance, contract service, and consumable
items.
All rates hourly. Fees are charged in whole hour increments with the minimum charge of 1 hour.
| Instrument | Internal User Rates | External User, Non-Profit Organization Rates | External User, For Profit Organization Rates |
| Nanomaterials Lab | $50 | $80 | $100 |
| Technical Assistance | $50 | $80 | $120 |