Department of Chemistry
Xiaohua Huang Research
Welcome to the homepage of Dr. Huang's Cancer Nanomedicine Research Group at The University of Memphis. We are working in an interdisciplinary area crossing chemistry, material science, biology, and medicine, with the ultimate goal of ending cancer. Our research interests are primarily in the development of novel inorganic nanomaterials and their bioconjugates for applications in biology and medicine. Of particular focuses are colloidal metallic nanoparticles such as spherical and rod shape gold nanoparticles and dual functional hybrid nanoparticles such as magnetic-plasmonic core-shell nanoparticles for cancer detection and treatment, including magnetic separation and optical detection of circulating tumor cells, single exosome profiling for blood-based early cancer detection and prediction of cancer metastasis, laser-based photothermal cancer therapy and combination treatment with chemotherapeutic drugs. Current major projects are outlined below.
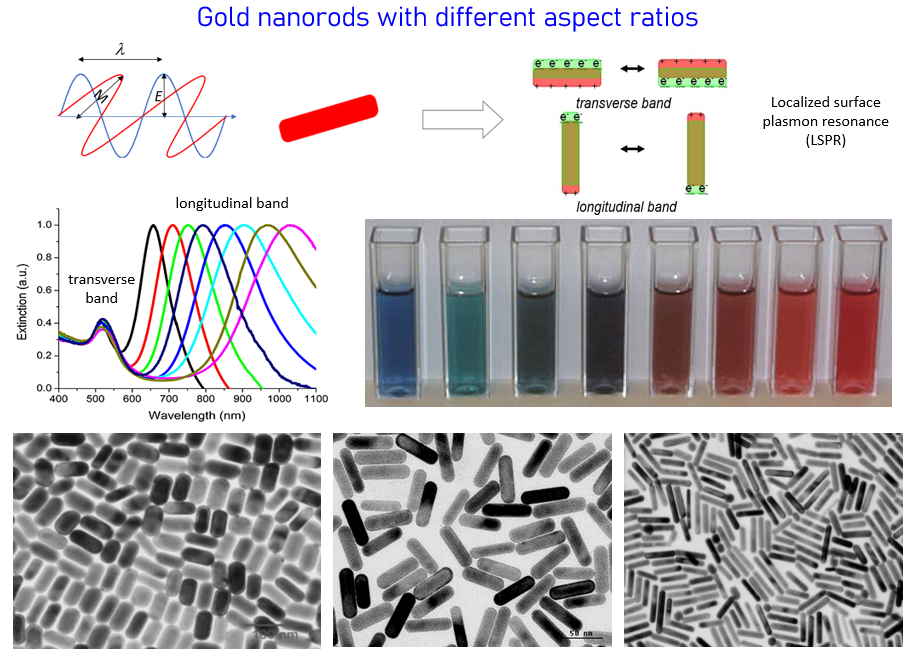
Multifunctional Gold Nanorods
Gold nanorods exhibit strong and tunable scattering and absorption properties in the near infrared region due to the unique physical geometry and localized surface plasmon resonance (LSPR). Research in our group is to explore these optical properties for a variety of biomedical applications from molecular imaging to cancer therapeutics. We synthesize and surface modify the nanoparticles with biocompatible polymers and cancer targeting ligands such as antibodies and peptides. Cancer diagnostic and therapeutic approaches include dark field microscopy, photothermal imaging, surface enhanced Raman spectroscopy, photodynamic and photothermal therapies.
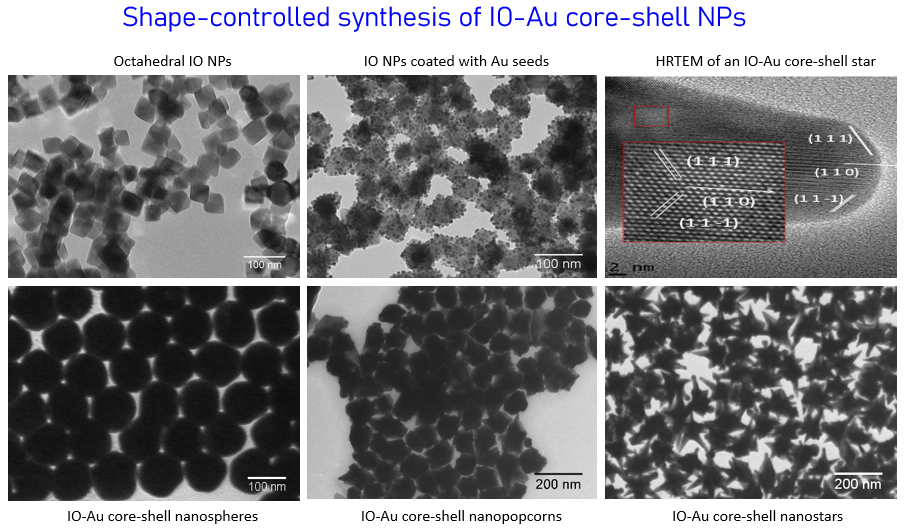
Anisotropic magnetic-plasmonic core-shell nanoparticles
Due to their high integrity, facile surface chemistry, excellent stability, dual functions from the core and shell, and the intrinsic shape-dependent optical properties from the metallic shell, anisotropic magnetic-plasmonic core-shell nanoparticles are very promising for applications in many areas including catalysis, energy conversion, biological separation, medical imaging, disease detection and treatment. However, the practical technological applications of this class of nanostructures are currently very limited, primarily due to the difficulties in their synthesis in different shapes, the lack of knowledge in the growth mechanisms and the structure-property relationships. We are trying to develop novel methods to make iron oxide-gold core-shell nanoparticles (IO-Au core-shell NPs) in different shapes, to understand the growth mechanisms and the magnetic/optical properties of the hybrid nanostructures. A number of experimental imaging and experimental approaches are used to characterize and study the nanoparticles. We have established collaboration with Prof. Yongmei Wang in Computational Chemistry to theoretically understand the optical properties of the hybrid nanomaterials and their effects by core size, shell thickness and the particle shape.
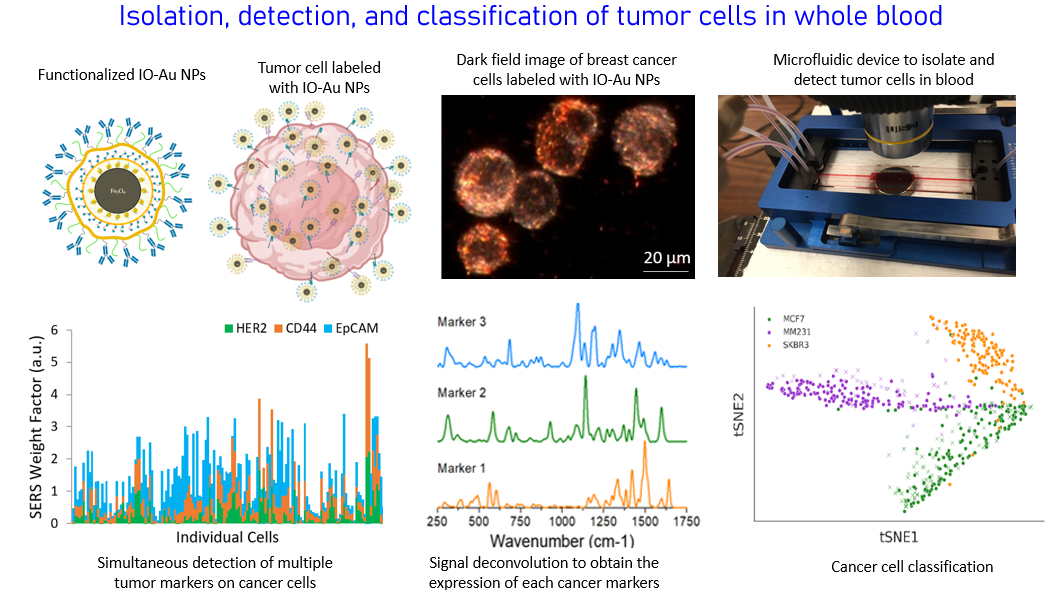
Capture, detection, molecular profiling, and classification of circulating tumor cells in whole blood
Metastasis is the main cause of death in cancer patients, accounting for 90% of the mortality. Metastasis is associated with the occurrence of malignant cells of epithelia origin in the blood which is called circulating tumor cells (CTCs). CTCs are a hallmark of invasive behavior of cancer, responsible for the development of metastasis. Their detection can provide a powerful tool in the clinic for diagnosis of metastasis at early stage, prognosis of cancer patients, assessment of tumor stage, monitoring of therapeutic response and ultimately helping optimization of treatment regimens. In addition, CTCs have been found in blood during early stages of tumorigenesis. Therefore, CTC assessment can be also used as a tool for early cancer detection. CTC detection, however, is difficult because of two major challenges: (1) CTCs are rare events, approximately one to few CTCs mixed with about 10 million leukocytes and 5 billion erythrocytes in 1 ml of blood of metastatic patients and (2) CTCs are a heterogeneous population due to tumor heterogeneity and potential changes of molecular characteristics during the epithelial to mesenchymal transition. We are developing new technology platforms to overcome these challenges based on dual functional magnetic-optical iron oxide-gold core-shell nanoparticles and microfluidic devices. Tumor cells in the blood can be captured and isolated from the blood cells through highly efficient immunomagnetic separation and subsequently detected with highly sensitive and specific surface enhanced Raman scattering spectroscopy, without the need of complicated sample processes and signal algorism. Simultaenous detection of different protein markers and cancer cells of different origins are achieved with multicolor magnetic-plasmonc iron oxide-gold core-shell NPs (IO-Au NPs) and signal deconvolution.
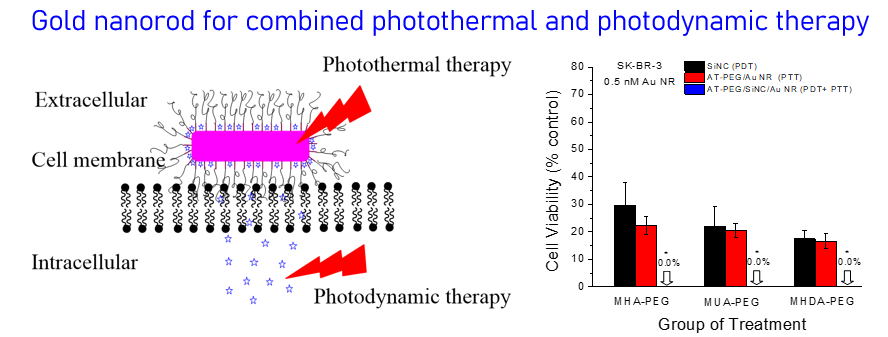
Combination cancer therapy
Combination cancer therapy is superior to individual treatment because of synergistic or additive effects. We are interested in developing novel drug-loaded nanocomplexes to combine chemotherapy with photothermal therapy or photodynamic therapy with photothermal therapy using near infrared-absorbing gold nanorod or iron oxide-gold core-shell nanocarriers. By finely controlling the surface chemistry, we aim to achieve highly efficient cancer treatment, which may prevent tumor recurrence.
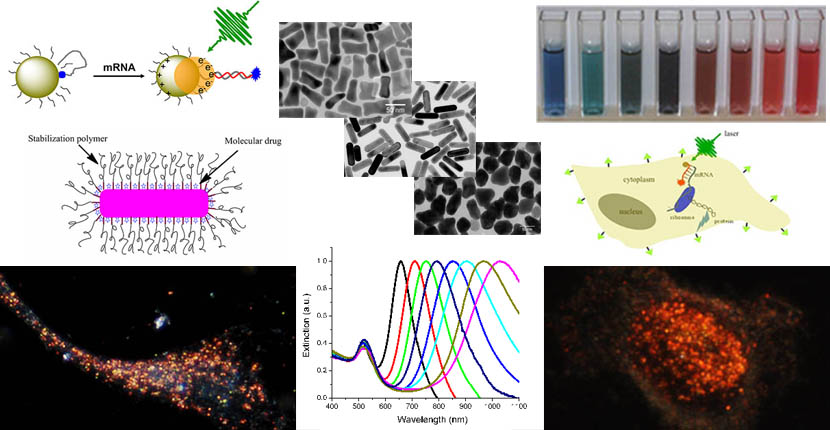
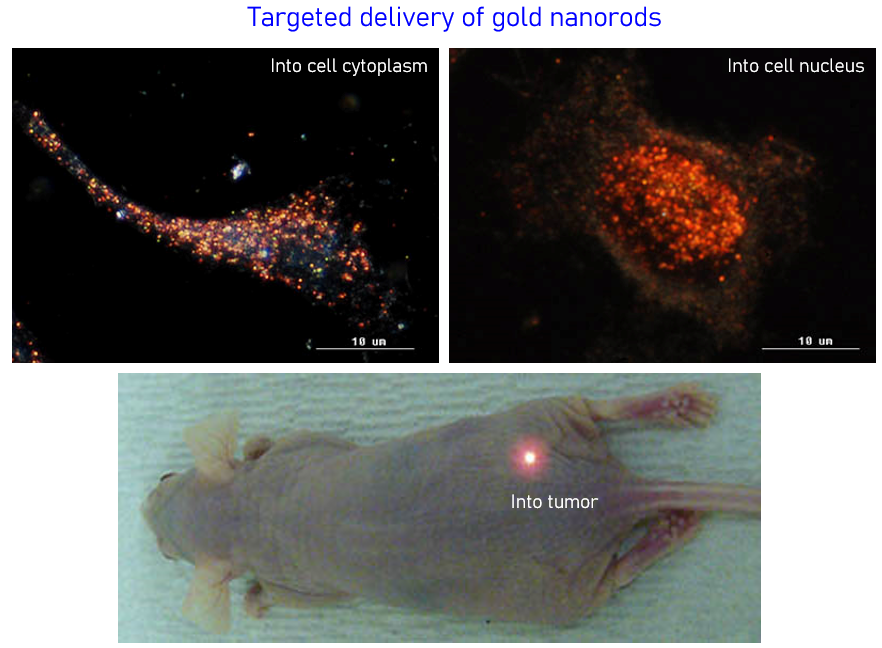
Targeted Drug Delivery
The targeted delivery of imaging and therapeutic agents into solid tumors is one of the most important and challenging problems in cancer nanomedicine, but the detailed mechanisms of nanoparticle delivery still remain a matter of debate. In our research, we use gold nanoparticles as tracers to investigate the mechanisms of active and passive tumor targeting and thus to maximize delivery efficiency and specificity of therapeutic agents such as SiRNA, antisense DNA and small molecule drugs. We are also interested in nuclear delivery of gene and peptides by designing and making cellular and nuclear penetrating gold nanoparticles.
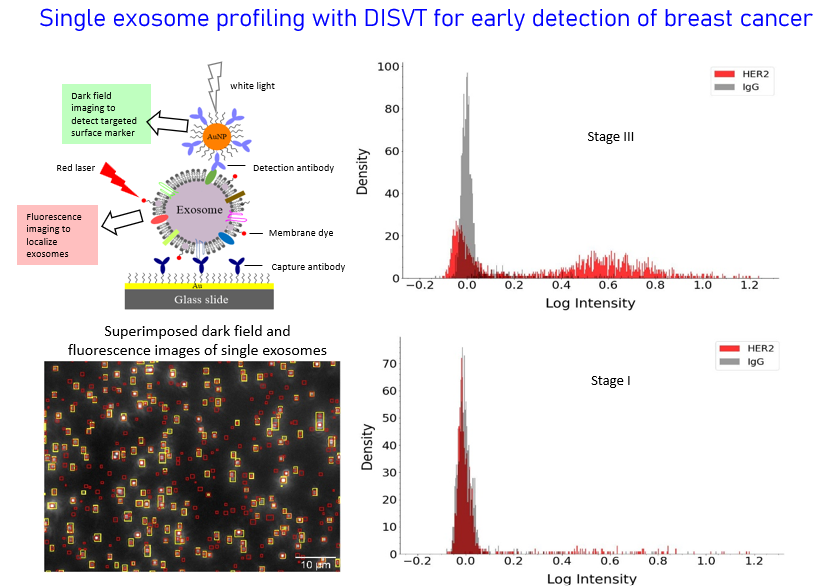
Single Exosome Profiling for Early Cancer Detection, Stage Differentiation, and Metastasis Prediction
Eearly cancer detetion is the key of effective treatment. Many types of cancer such as ovary cancer and pancreatic cancer do not have screeing techniques for early cancer detection. Metastasis is the major cause of cancer mortality, accounting for over 90% of cancer-related deaths. Prediction of the risk of metastasis at the time of cancer diagnosics or after surgery remains an unmet challenge in the clinic. The goal of our research is to address these two key challenges to help fighting cancer by development of new generation liquid biopsy with the molecular analysis of single extracellular vesicles particually exosomes in plasma. Exosomes are 30-200 nm membrane-bound vesicles of endocytic origin and secreted continuously by nearly all cells. They carry molecular constitutes of their parental cells and are found in many body fluids such as plasma, saliva, and urine. Thus, cancer cells can be detected through their exosomes. Recently, we developed a dual imaging single vescile technology (DISVT) that can detect and profile surface protein markers on single exosomes. Using this technology, we have shown that our DISVT, but not traditional bulk ELISA method, can detect breast cancer at early-stage. Currently, we are using this technology to explore the abilities of plasma exosomes for differentiation of breast cancer at different stages and prediction of breast cancer metastasis.